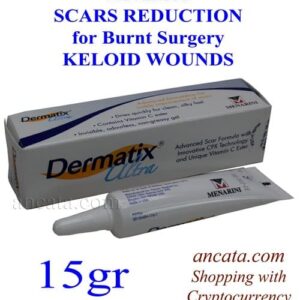
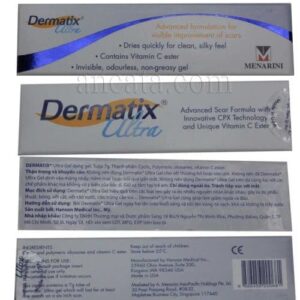
Salonpas Liniment Pain Relief and Anti-Inflammatory Solution (50ml) – Free Ship
Salonpas Liniment, manufactured by Hisamitsu Pharmaceutical, is a topical analgesic solution designed to alleviate pain and inflammation associated with musculoskeletal conditions such as muscle pain, fatigue, shoulder stiffness, back pain, bruises, sprains, muscle cramps, rheumatoid arthritis, and bone injuries. The product is formulated as a 50ml roll-on liquid for external application.
Nhận HSV Point cho mỗi lần mua
Cam kết hàng chính hãng
Miễn phí giao hàng, tối đa 44K
Đổi trả hàng trong vòng 7 ngày












Reviews
There are no reviews yet.